Phosphor Development Addresses Lower Efficacy of Warm-White LEDs
投稿人:电子产品
2014-05-20
The majority of today’s lighting standard “white” LEDs use an InGaN semiconductor, emitting light in the blue part of the spectrum, combined with a yttrium aluminum garnet (YAG) phosphor (doped with cerium). Most of the LED’s blue photons are absorbed by the phosphor and re-emitted in the yellow part of the spectrum. The mix of the residual blue photons and yellow illumination provides a good approximation of white light to the eye.
This combination of LED and phosphor is a proven technology with good performance, but it is not perfect. One notable deficiency is a limitation to high correlated color temperatures (CCT) and lower color rendering indices (CRI) due to a lack of a red component in the system’s output. Combining the YAG phosphor with other materials increases red emissions, leading to the availability of “warm” white LEDs, but at a cost of hard-won efficacy.
This article describes the problem in detail, explores how manufacturers of phosphors for LED lighting are rising to the challenge, and then considers what impact their phosphor research will have on the efficacy, CCT, and CRI of solid-state illumination.
Blue LED plus YAG phosphor equals white light
The phosphor used for high-brightness LEDs is a highly specialized material comprising YAG doped with the rare-earth-element cerium (scientifically labeled as Y3Al5O12:Ce3+). The material was evolved from the phosphor used for CRTs and was first synthesized in 1967.
In operation, the LED’s blue photons are absorbed by atoms or molecules in the YAG phosphor, raising the constituent electrons to a higher energy level. The electrons then rapidly fall back to a lower level by re-emitting the energy partially as a photon of a longer wavelength (a little red, some green, and a lot of yellow) than the absorbed photon and partially as heat. (See the TechZone article “Whiter, Brighter LEDs.”)
The primarily yellow emission from YAG phosphor and the blue radiation directly from the LED that “leaks” through a YAG:Ce coating combines to give a good approximation of white light.
The phenomenon behind the transformation of blue light to “white” light by absorption and re-emission from phosphor is known as Stokes shift, named after Irish physicist George G. Stokes who described the effect in an 1852 paper. Figure 1 shows how the Stokes shift occurs via the absorption and emission curves for YAG phosphor.
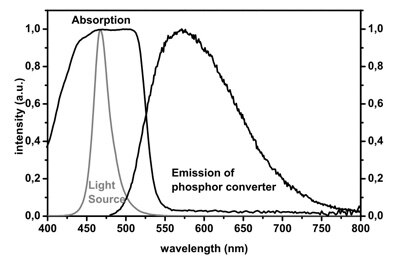
Figure 1: Absorption and emission spectra for YAG phosphor exposed to blue light.¹
Figure 2 shows the relative spectral emission curve for a modern white LED using YAG phosphor (in this case an OSRAM OSLON SSL 150 LED producing 136 lm (forward current 350 mA, forward voltage 3.1 V) at an efficacy of 125 lm/W. The dashed line is the eye’s sensitivity function and illustrates how the eye reacts to different wavelengths of light. Although the first peak (corresponding to blue photons directly from the LED) is larger, it is less noticeable because the eye is not very sensitive to light of this wavelength. (See the TechZone article “Royal Blue LEDs: Decoding the Datasheet.”) In contrast, the photons emitted by the phosphor are centered on 560 nm — the point at which the eye best senses light.

Figure 2: Spectral emission curve for a modern white LED (OSRAM OSLON SSL 150).
Because it is a proven technology producing acceptable results, the properties of YAG represent a benchmark for other LED phosphors. Not only does YAG phosphor strongly absorb blue photons, it also has a fast “decay time” (the time it takes for an electron that has absorbed a blue photon to “give up” the energy as a yellow photon and heat) that prevents a phenomenon known as saturation quenching. This is a process whereby normally emitted photons are “overwhelmed” and prevented from escaping by a high photon flux within the phosphor matrix.
YAG phosphor has a number of other advantages for LED applications. First, the quantum efficiency (a measure of the total output photon energy from the phosphor compared to the input photon energy from the LED) of YAG phosphor under blue LED excitation is around a healthy 80 percent. Second, researchers report that there are no indications that YAG phosphor degrades under long-term blue LED excitation or exposure to moisture. Lastly, the synthesis of YAG phosphor is relatively straightforward and uses the same high-purity precursors (Y2O3, Al2O3, CeO2) that have been qualified for many years for use in traditional CRT phosphors.²
The drawbacks of YAG phosphor
Despite its proven performance for LEDs though, YAG phosphor is not perfect. Two practical problems are “temperature quenching” and relatively low chemical stability.
The causes of temperature quenching are tricky to grasp, but in layman’s terms, elevated temperatures cause the electrons that would normally absorb the blue photon (and then emit a yellow one) to “go missing” (in other words the atoms are ionized). YAG phosphor suffers from temperature quenching at around 200°C, not unusually high for an LED during normal operation.
Moreover, the material’s low chemical stability puts a limit on LED lifetime (defined as the point at which the device’s luminosity has declined to less the 70 percent of its output when new). Manufacturers counter this argument by pointing out that modern white LEDs are capable of operating for 30,000 hours or even longer, but researchers suggest that this is measured under “ideal” operating conditions and the chemical instability could compromise performance for devices used in harsh environments.
However, perhaps the biggest challenges for YAG phosphor are aesthetic ones. Solid-state lighting makers are keen for consumers to embrace the technology, but one common complaint is that white LEDs produce a “harsh” light with little of the “warmth” delivered by traditional incandescent lighting. This perception is due to the fact that YAG phosphor produces a “bluish” white light with little red content. The result is light with a high CCT (5000 to 8300 K)
The lack of significant red wavelengths introduces another problem for YAG phosphor-powered devices: poor CRI. CRI is a measure of how well an illumination source reproduces an object’s color compared to sunlight (which has a CRI of 100.) Despite their other obvious drawbacks, incandescent bulbs have a CRI of around 95. In comparison, cool-white LEDs typically have a CRI of 70 to 80. (See the TechZone articles “Defining the Color Characteristics of White LEDs” and “What Is the Color Rendering Index and Why Is It Important?” for more on CCT and CRI.)
LED makers have addressed the CCT and CRI challenges to some extent by mixing the YAG phosphor with another phosphor that adds red wavelengths, extending CCT to warmer zones and improving CRI. Substituting a blue LED for an ultraviolet one can make further improvements to CCT and CRI. LED makers offer commercial ultraviolet products for this purpose. For example, Philips Lumileds offers an ultraviolet version of its Luxeon LED. The device emits light of a wavelength between 395 and 400 nm and offers a minimum radiant intensity of 525 mW/sr at 500 mA. (For reasons to do with the eye’s inability to see ultraviolet light, ultraviolet LEDs are rated in terms of radiant intensity (watts/steradian) rather than the more familiar efficacy (lm/W) figures quoted for white LEDs.)
The major drawback of adding the “red” phosphor is a reduction in LED efficacy. Worse yet, the red phosphor’s temperature-quenching threshold is even lower than YAG phosphor, further compromising efficacy at typical LED operating temperatures.
Cree’s Xlamp® XT-E white LEDs operate at 350 mA and 2.85 V, but the cool-white (5000 K) version boasts a luminosity of 135 lm and an efficacy of 135 lm/W, while the warm white (2700 K) comes in at 97 lm and 97 lm/W, respectively. Other manufacturers’ warm-white products exhibit similar lower efficacies compared with their cool-white devices.
Figure 3 illustrates the difference between CCTs and CRIs for a blue LED and YAG phosphor and an ultraviolet LED combined with a mix of YAG phosphor and a red phosphor.
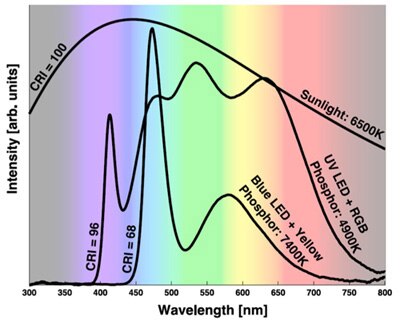
Figure 3: Combining an ultraviolet LED with yellow/red phosphor improves CCT range and CRI but at the expense of efficacy.³
Improving phosphor efficacy
The pursuit of LED efficacy has been a key driver of solid-state lighting component development for the major manufacturers over the last decade-and-a-half. Dramatic improvements have been made both in the initial generation of photons and then in their extraction from the die. (See the TechZone article “Material and Manufacturing Improvements Enhance LED Efficiency.”) The last thing the manufacturers want is for the relatively poor conversion efficiency of the red phosphor to compromise the luminosity of their warm-white LEDs.
Consequently, phosphor manufacturers are stepping up to the challenge to produce new materials that promise to form commercial phosphors with higher conversion efficiency while supporting a wide CCT range and good CRI. Among the most promising candidates are nitride and oxynitride materials that replace the electroluminescent properties of cerium with another rare element, the metal Europium (Eu).
Many of these new phosphors can be excited with violet or blue LEDs and match both the room and high-temperature quantum efficiency of YAG:Ce phosphor. In addition, the nitride phosphors do not degrade under high-temperature/high-humidity conditions, making them suitable for LED lighting used in harsh environments.
One family of efficient oxynitride phosphors is the MSi2O2N2:Eu2+ (where M = Ca2+, Sr2+, Ba2+) compositions whose emission ranges from 575 to 675 nm with a quantum efficiency of greater than 85 percent at a temperature exceeding 200°C (Figure 4). The yellow, orange, and red Eu2+ emissions from these nitride phosphors can be combined with YAG:Ce for warm-white LEDs of higher efficacy than current commercial products. Phosphor blends of YAG:Ce and CaAlSiN3:Eu2+ combined with blue LEDs have already been used to manufacture high-CRI warm-white lamps.
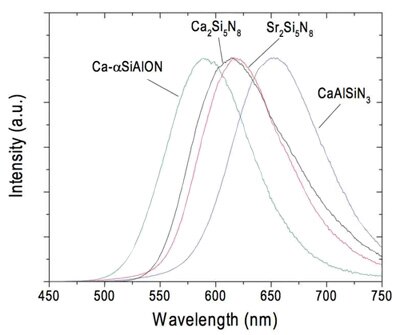
Figure 4: Nitride and oxynitride materials show promise as high-quantum-efficiency phosphors for warm-white LEDs.²
One drawback is that while these new materials have significant potential, synthesizing them is much more difficult than for traditional phosphors. However, that has not stopped some enterprising manufacturers from commercializing nitride phosphors. Intematix added red nitride materials to its phosphor portfolio in 2011. The company’s phosphor targets efficient warm-white LEDs for general lighting with the added bonus for customers of freeing them from some patent licensing issues.
The company claims that the new phosphor will allow the lighting market to create warm-white applications with much higher efficacy and better CRI (up to 98) than traditional YAG phosphor solutions. Use of the new phosphors is said to enable customers to (legally) circumvent certain patents pertaining to the application of YAG phosphor to an LED package that would otherwise attract a licensing fee.
High-efficiency warm-white LEDs coming soon
LEDs have seen dramatic increases in efficacy as manufacturers seek to convince consumers that solid-state lighting is a practical alternative to traditional illumination. However, further gains in photon production and light extraction are proving hard to find, so LED makers are increasingly turning their attention to other aspects of a chip’s characteristics in search of better performance.
One such aspect is phosphors, particularly those used to add red wavelengths to white LEDs’ output in order to make the devices appear warmer. Conventional solutions extend a product’s CCT and CRI, meeting consumer demand for a wide range of temperature choices and faithful color reproduction, but negate some of the hard-won efficacy gained from the introduction of other technologies.
Work on a new range of phosphors, based on nitrides and oxynitrides doped with Europium, promises to yield new materials offering good CCT and CRI, but with quantum efficiencies on par (or better) with the pure YAG phosphor used in contemporary, highly efficient cool-white LEDs. Some of the materials are now being introduced into the market and engineers should expect a new generation of more efficient warm-white LEDs to follow soon.
For more information about the devices described in this article, use the links provided to access component details on the DigiKey website.
References
- “Studies on Luminescence and Quenching Mechanisms in Phosphors for Light Emitting Diodes,” Volker Michael Bachmann, University of Utrecht
- “Phosphors for LED-based Solid-State Lighting,” Anant A. Setlur, Interface, The Electrochemical Society, Winter 2009.
- “What Are the Limits for SSL?,” Shuji Nakamura, University of California, Santa Barbara, February 2010.
免责声明:各个作者和/或论坛参与者在本网站发表的观点、看法和意见不代表 DigiKey 的观点、看法和意见,也不代表 DigiKey 官方政策。







 中国
中国